
For this reason, the Hückel method is limited to planar systems. Humanities Anthracene is a yellow, crystalline solid found in coal tar. While MO theory deals with sigma bonds as well as pi bonds, we will restrict. This is referred to as sigma-pi separability and is justified by the orthogonality of \(\sigma\) and \(\pi\) orbitals in planar molecules.

Resonance energy for benzene, naphthalene, anthracene, phenanthrene. There are three benzene rings consisting of 1 sigma and 2 pi bonds. Resonating structures with greater number of covalent bonds (sigma and pi bonds). The method limits itself to addressing conjugated hydrocarbons and specifically only \(\pi\) electron molecular orbitals are included because these determine the general properties of these molecules the sigma electrons are ignored. Anthracene fullerene is a polycyclic aromatic hydrocarbon. Lets count all the sigma and pi bonds in the anthracene structure. The to transition requires an absorption of a photon with a wavelength which does not fall in the UV-vis range (see table 2 below). The wavefunctions used to describe the bonding orbitals in each framework results from different combinations of atomic orbitals. (pi to pi star transition) n- (n to pi star transition) - (sigma to sigma star transition) n - (n to sigma star transition) and are shown in the below hypothetical energy diagram. Within the Hückel approximation, the covalent bonding in these hydrocarbones can be separated into two independent "frameworks": the \(\sigma\)-bonding framework and the the \(\sigma\)-bonding framework. The Hückel approximation is used to determine the energies and shapes of the \(\pi\) molecular orbitals in conjugated systems. (Note: by convention, in planar molecules the axis perpendicular to the molecular plane is the z-axis.) (CC BY-NC Ümit Kaya via LibreTexts) (b) One singly occupied unhybridized 2p z orbital remains on each carbon atom to form a carbon–carbon \(π\) bond. A benzene rings conjugated double bonds peak primarily at 180 and 200 nm. A single bond (sigma bond) would absorb around 130 nm. This uses 10 of the 12 valence electrons to form a total of five \(σ\) bonds (four C–H bonds and one C–C bond). UV light is in the range of about 10-400 nm. : (a) The σ-bonded framework is formed by the overlap of two sets of singly occupied carbon sp 2 hybrid orbitals and four singly occupied hydrogen 1s orbitals to form electron-pair bonds. Thus each carbon forms a set of three \(\sigma\) bonds: two C–H ( sp 2 + s) and one C–C ( sp 2 + sp 2) (part (a) of Figure 10.5.1 This angle suggests that the carbon atoms are sp 2 hybridized, which means that a singly occupied sp 2 orbital on one carbon overlaps with a singly occupied s orbital on each H and a singly occupied sp 2 lobe on the other C. Experimentally, we know that the H–C–H and H–C–C angles in ethylene are approximately 120°. The original thread will be attended by our experts shortly. The simplest hydrocarbon to consider that exhibits \(\pi\) bonding is ethylene (ethene), which is made up of four hydrogen atoms and two carbon atoms. Subject: Chemistry, asked on 1/7/18 How and how many pi and sigma bonds are there in anthracene Share with your friends 0 Follow 0 Customer Support, Meritnation Expert added an answer, on 1/7/18 Dear Student, It seems that you have posted this question twice on the forum. This is, in fact, a more sophisticated version of a free-electron model. The bonds C1C2, C3C4, C5C6 and C7C8 are about 136 pm in length, whereas the other carboncarbon bonds are about 142 pm long. An approximation introduced by Hückel in 1931 considers only the delocalized p electrons moving in a framework of \(\pi\)-bonds. In naphthalene, the carboncarbon bonds are not the same length. In aromatic hydrocarbons they have a) Only sigma bonds b) Only pi bonds c) a sigma and delocalized pi bond d) a sigma and two pi bond Answer- c) a sigma and delocalized pi bond 2. A sigma bond, sigma, resembles a similar 's' atomic orbital.
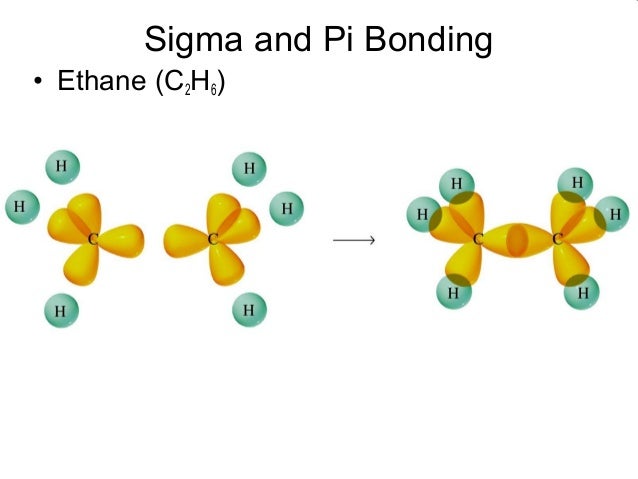
Both acquired their names from the Greek letters and the bond when viewed down the bond axis. Sigma bonds are formed by end-to-end overlapping and Pi bonds are when the lobe of one atomic orbital overlaps another. Molecular orbital theory has been very successfully applied to large conjugated systems, especially those containing chains of carbon atoms with alternating single and double bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals.
#Anthracene sigma and pi bonds full
Demonstrate how Hückel's theory approximates the full molecular orbital picture of molecules by treating the \(\sigma\)-bonding and \(\pi\)-bonding networks independently.


 0 kommentar(er)
0 kommentar(er)
